Abstract. Metabolic dysfunction–associated steatotic liver disease (MASLD) is the most common metabolic liver disease in the world, affecting approximately a quarter of the adult population of the world [1]. Considerable attention is paid to the study of MASLD, taking into account the comorbidity with arterial hypertension (AH), which affects 30-35% of the adult population of the world [2]. Currently, numerous studies are being conducted to determine the pathophysiological mechanisms of the cardiovascular pathology development in patients with a comorbid course of MASLD and AH [3]. It is known that MASLD is considered to be one of the diseases associated with metabolic disorders, including violations of carbohydrate metabolism, coupled with obesity and insulin resistance (IR) [4]. It is now evident that MASLD, generally perceived as a benign condition, may have on the contrary an important deleterious impact for diabetic patients [5]. The presence of MASLD is believed to be associated with cardiovascular disease (CVD) increased risk. Scientists have suggested a hypothesis that MASLD is not only CVD marker, but it may also be involved in its pathogenesis [6]. MASLD and CVD association mechanisms were also investigated, including oxidative stress development, systemic inflammation, IR and hyperlipidaemia [7]. Thus, the analysis of IR indicators is an important component of the MASLD and AH diagnosis.
Objective: to explore the relationship between MASLD on the background of AH and IR indicators including the levels of fasting glucose, insulin and insulin resistance index (HOMA-IR).
Materials and methods. 102 patients with MASLD were studied, who were divided into the following groups: the main group – 40 patients with comorbid course of MASLD and AH; the comparison group – 42 patients with isolated course of MASLD; the control group – 20 relatively healthy individuals.
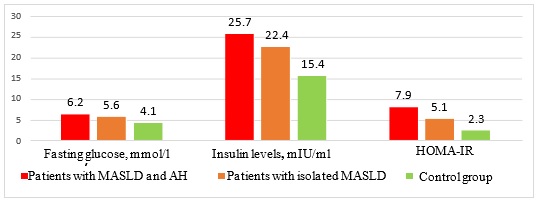
Results. Comparative analysis of IR indicators (Figure 1) revealed significantly higher insulin levels in patients with comorbid course of MASLD and AH than in the group with isolated MASLD and the control group. Thus, the insulin levels in patients with comorbid pathology was (25.7±4.5) mIU/ml, in the group of patients with isolated MASLD – (22.4±3.8) mIU/ml, and in the control group – (15.4±4.1) mIU/ml (p1˂0.01, p2˂0.05). The average values of the HOMA-IR were: (7.9±0.6) in the group with comorbid pathology, (5.1±0.5) in the group with isolated course of MASLD and (2.3±0.05) in the control group (р1˂0.05, р2=0.05). The average values of fasting glucose levels among the examined patients were as follows: (6.2±1.3) mmol/l in the group with comorbid pathology, (5.6±1.1) mmol/l in the group with isolated course of MASLD and (4.1±0.8) mmol/l in the control group.
Fig. 1 Average values of carbohydrate metabolism indicators in the groups of studied patients
Discussion. Analysing the results of the carbohydrate status in the examined patients, a relationship was found between the presence of concomitant AH in patients with MASLD and the general deterioration of the carbohydrate status. Thus, a significant increase in the level of insulin, HOMA-IR index and fasting glucose in patients with comorbid MASLD and AH compared with the group of patients with isolated MASLD and the control group confirms the independent role of AH in the development of IR in patients with MASLD. The results of our study do not contradict the data of the world literature, confirming that the development of IR is one of the key factors underlying the pathogenetic mechanism of the relationship between MASLD and AH [8-10].
Conclusions. A significant increase of insulin levels and HOMA-IR in patients with comorbid course of MASLD and AH compared to the group of patients with isolated MASLD and the control group confirms the independent role of AH in the IR development in patients with MASLD. Further studies that consider the role of IR in the development of MASLD are needed to determine therapeutic approaches for the management and treatment of this cohort of patients.
References
1. Eslam M., Sanyal A.J., George J.; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158(7):1999-2014.e1
2. Duell P.B., Welty F.K., Miller M., et al. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(6):e168-e185. doi:10.1161/ATV.0000000000000153
3. Del Villar-Carrero R.S., Blanco A., Ruiz L.D., et al. Bridging Metabolic-Associated Steatotic Liver Disease and Cardiovascular Risk: A Potential Role for Ketogenesis. Biomedicines. 2024;12(3):692. Published 2024 Mar 20. doi:10.3390/biomedicines12030692
4. Samuel V.T., Shulman G.I. Cell Metab 2018; 27(1): 22-41. doi: 10.1016/j.cmet.2017.08.002
5. Loomba R., Friedman S.L., Shulman G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537-2564. doi:10.1016/j.cell.2021.04.015
6. Motamed N., Rabiee B., et al. Clin Res Hepatol Gastroenterol 2017; 41(1): 31-38. doi: 10.1016/j.clinre.2016.07
7. Leach N.V., Dronca E., et al. Eur J Intern Med 2014; 25(8): 762-767. doi: 10.1016/j.ejim.2014.09.007.
8. Watt M.J., Miotto P.M., De Nardo W., Montgomery M.K. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr Rev. 2019;40(5):1367-1393. doi:10.1210/er.2019-00034
9. Sakurai Y., Kubota N., Yamauchi T., Kadowaki T. Role of Insulin Resistance in MAFLD. Int J Mol Sci. 2021;22(8):4156. Published 2021 Apr 16. doi:10.3390/ijms22084156
10. Fujii H., Kawada N., Japan Study Group Of Nafld Jsg-Nafld. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2020;21(11):3863. Published 2020 May 29. doi:10.3390/ijms21113863.
|