Plants are promising sources of substances with a wide range of activities, including antimicrobial, which may help to overcome antibacterial resistance. Unfortunately, many plants are toxic to human organism, which makes their use for therapeutic purposes impossible [1]. Therefore, testing the toxicity of plant extracts is an important preclinical stage in the investigations of new antimicrobial drugs. The aim of this research was to study the acute toxicity of ethanolic Ruta graveolens L. extracts.
The acute toxicity of garden ruta (GR) herb extracts was studied in the conditions of the clinical and biological experimental base of IFNMU. The research was conducted by the national “General Ethical Principles of Animal Experiments” (Ukraine, 2001), which corresponds to the provisions of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986) [2, 3].
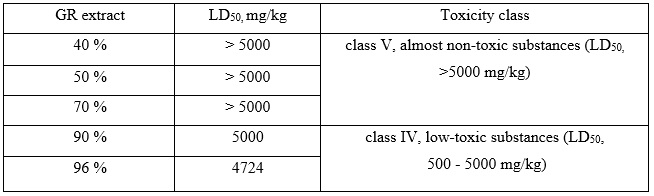
Study of acute toxicity of 40%, 50%, 70%, 90% and 96% ethanolic ruta herb extracts were conducted on white non-linear male mice weighing 18-35 grams, which were standardized according to physiological and biochemical parameters and were on a standard diet. The investigated plant extracts were administrated intragastrical to the animals at a dose of 5000 mg/kg, which corresponds to the maximum dose of the toxicity class IV (low-toxic substances). If the animals died, an additional 500 mg/kg was administered – the maximum dose of toxicity class III (moderately toxic substances) [4, 5]. The animals were divided into six groups. Animals of the I group were given drinking water, and animals from II-IV groups – ruta herb ethanolic extracts. During the experiment, an additional group was formed (group VII), for animals from this group, a 96% Ruta graveolens extract was given in a dose of 500 mg/kg. The animals were observed for 14 days. The degree of toxicity was assessed by changes in general condition and mortality. The toxicity class was determined according to the generally accepted classification [4]. The results of a study on the acute toxicity of ruta herb ethanolic extracts are presented in Table 1.
Table 1. The results of a study on the acute toxicity of ruta herb ethanolic extracts
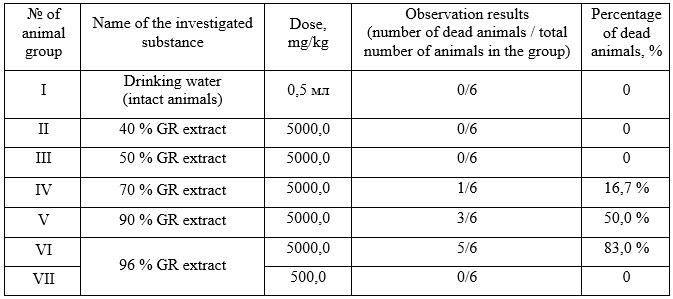
The data indicates that during the entire period of observation among animals of groups I, II and III, no deaths of animals were registered. In other studied groups, the death of animals was recorded after the introduction of ruta extracts: in the IV group (70% extract) - 1 animal - on the 6th day, in the V group (90% extract) - 3 animals - on the 2nd, 4th and 6th days, in VI group (96% extract) - 5 animals - on the 2nd and 3rd days. Considering the death of many animals in group VI, a new group of animals was additionally formed, which were injected with a 96% ethanolic ruta herb extract at a dose of 500 mg/kg. During 14 days of observation, the death of animals in the VII group was not registered. Animals of I - IV and VII experimental groups were neat, active, and responded to sound and light stimuli, urination and defecation processes were normal, breathing disorders and convulsions were not observed, and reflex excitability was preserved. In animals from groups V and VI, during the first 5 days, there was observed a decrease in motor activity, drowsiness, photophobia, and decreased appetite, the wool was slightly moistened.
Studies on the acute toxicity of 40%, 50% and 70% Ruta graveolens L. ethanolic extracts showed the absence of their toxic effect after a single intragastric administration to white mice at a dose of 5000 mg/kg, which indicates that the lethal dose LD50 is higher than the one given to the animal and allows them to be classified as toxicity class V (almost non-toxic substances). For the 96% GR extract, additional calculations were performed to establish the LD50 (Tab. 2) [6].
Table 2. Parameters of acute toxicity of Ruta graveolens L. ethanolic extracts
In conclusion, we have established that 40%, 50% and 70% ethanolic extracts of Ruta graveolens L. can be classified as almost non-toxic substances (toxicity class V, LD50, > 5000 mg/kg), and extracts on 90%, 96% ethanol – as low-toxic substances (toxicity class ІV, LD50, 500 - 5000 mg/kg), according to toxicity classification.
References
1. Abreu A.C., McBain A.J., Simoes M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. – 2012; Vol.29, №9: 1007-1021 p. Доступно: doi: 10.1039/c2np20035j.
2. Order of the Ministry of Health of Ukraine No. 944 dated 12/14/2009. "On approval of the Procedure for conducting preclinical studies of medicinal products and examination of materials for preclinical studies of medicinal products". (In Ukrainian)
3. Commission of the European Communities: Council Directive of 18 December 1986 on the Lows, regulating the Application of Principles of Good Laboratory Practice and the Verification of Their Applications for Tests on Chemical Substances (87/18/EEC). The Rules Governing Medicinal Products in the European Community. 1991. V. 1. P. 145 – 146.
4. Preclinical studies of medicinal products: [methodological recommendations] / Edited by O. V. Stefanov. K.: Avicenna, 2001. 528 p. (In Ukrainian)
5. Nykyforuk A. Ya., Fira L. S., Lykhatskyi P. H. Experimental substantiation of the safety of a thick extract from garden spinach. Ukrainian Biopharmaceutical Journal. Kharkiv, 2018. 3(56). P. 16-21. https://doi.org/10.24959/ubphj.18.180. (In Ukrainian)
6. Singh, M., Al-Khatri, S., El-Shamaa, K., and Niane, A.A.. Statistical Design and Analysis of Date Palm Insect Pest Management Experiments. In M. El Bouhssini and J.R. Faleiro (Editors), Date Palm Pests and Diseases Integrated Management Guide. Abu Dhabi, 2018. P. 20-39. ISBN 978-9291275052.
|