Introduction. Diabetes mellitus is a pathology that is associated with a violation of all types of metabolism. It is known that the insufficient influence of insulin leads to a decrease in the activity of the pyruvate dehydrogenase complex. One of the manifestations of the disease is lactic acidosis [2, p. 59]. This leads to a strain on the buffer systems of the blood, in particular the bicarbonate buffer system [3, p. 12].
Alloxan monohydrate modulates events similar to type 1 autoimmune diabetes. Its toxicity is associated with the development of oxidative stress. Beta cells of the Langerhans islets are destroyed due to low antioxidant protection [5, p. 118172].
Hyperglycemia, which occurs as a result of insulin deficiency, worsens the pro-oxidant/antioxidant situation in the body, increasing the formation of free radicals. This depletes the glutathione defense system that was previously destroyed by alloxan monohydrate.
Melatonin is an adaptogen that has antioxidant properties and has an antiglycemic effect [1, p. 236].
How melatonin affects the blood buffer systems of rats with alloxan diabetes is still poorly understood.
The aim of the work was to establish changes in blood buffer systems and blood lactate dehydrogenase activity of rats with alloxan diabetes under the influence of melatonin.
Materials and methods. The experiments were carried out in accordance with established norms for the treatment of experimental animals. Thirty white outbred male rats weighing 150 grams were used in the work. Diabetes was induced by a single administration of alloxan monohydrate at the rate of 170 mg per kg of the animal's body weight. The rats were divided into three groups: intact, diabetic, and diabetic with melatonin. Melatonin was administered daily in the morning at the rate of 10 mg per kg of body weight for a week. Animals were decapitated on the twelfth day after the start of the experiment in accordance with the established ethical standards. Lactate dehydrogenase activity, fasting glucose level and blood pH were determined according to standard methods.
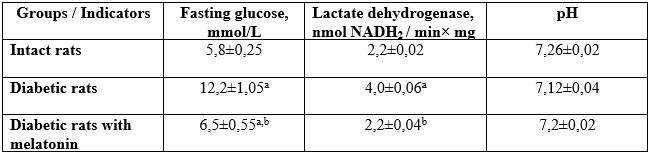
The results. Administration of alloxan monohydrate led to the death of some animals. The rest formed a group of diabetic animals with a fasting glucose level 105% higher than that of intact animals.
Changes in blood buffer indicators of rats with alloxan diabetes under the conditions of melatonin administration (n=6, x±Sx):
Table 1
Note:
1. a, b - changes are reliable (р≤0,05).
2. a - concerning intact rats;
b - concerning rats with diabetes mellitus.
Lactate dehydrogenase activity in diabetic animals was twice higher than that of the intact group. In this group of rats, the pH was slightly shifted to the acidic side compared to intact animals. It is possible that the bicarbonate buffer system is activated, which interacts with excess lactate, forming a sodium salt. Administration of melatonin led to a 40% decrease in fasting glucose, normalization of lactate dehydrogenase activity and blood pH in alloxandiabetic rats compared to the parameters of intact animals. Melatonin activates glucose transporters and activates aerobic transformation processes.
Melatonin stimulates the expression of glucose-6-phosphate dehydrogenase genes [4, p. 3018]. This ensures the supply of NADPH2 for the reduction of glutathione with its subsequent use in the way of neutralization of hydrogen peroxide. This prevents the formation of hydroxyl radical and development of free-radical reactions of biomolecules destruction.
Conclusion. Melatonin leads to the normalization of buffer systems functioning in the blood of rats with alloxan diabetes, possibly due to the restoration of absorption and use of glucose by the aerobic pathway, which reduces the level of lactate.
References
1. Alexander Panossian, Thomas Brendler The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections Pharmaceuticals (Basel) (2020) 8;13(9):236. doi: 10.3390/ph13090236.
2. Bhat, Javaid Ahmad; Masoodi, Shariq Rashid et al. Lactic Acidosis in Diabetic Ketoacidosis A Marker of Severity or Alternate Substrate for Metabolism Indian Journal of Endocrinology and Metabolism (2021) 25(1):p 59-66, DOI: 10.4103/ijem.IJEM_753_20
3. Jorge Lorenzo Calvo, Huanteng Xu, Daniel Mon-López, Helios Pareja-Galeano & Sergio Lorenzo Jiménez Effect of sodium bicarbonate contribution on energy metabolism during exercise: a systematic review and meta-analysis. Journal of the International Society of Sports Nutrition (2021) volume 18, Article number: 11, P.17. https://doi.org/10.1186/s12970-021-00410-y
4. Marek Samec, Alena Liskova, Lenka Koklesova Metabolic Anti-Cancer Effects of Melatonin: Clinically Relevant Prospects. Cancers (Basel) (2021);13(12):3018. doi: 10.3390/cancers13123018.
5. Priyankar Dey The role of gut microbiome in chemical-induced metabolic and toxicological murine disease models. Life Sciences (2020) Volume 258, 118172. https://doi.org/10.1016/j.lfs.2020.118172
|