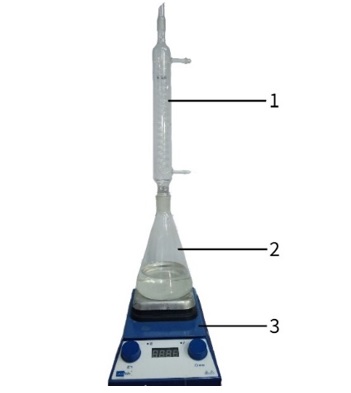
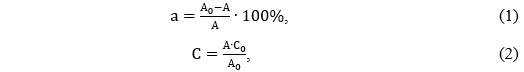Synthesis methodology. The synthesis of zinc oxide was carried out using the sol-gel method (Fig. 1). To do this, 10 g of zinc acetate was dissolved in 300 ml of ethanol at 80°C and under constant stirring for 10 hours to obtain a clear solution. The resulting solution was then cooled to 0 °C and NaOH solution (0.225 mol/L) was added dropwise to form a white gel. The resulting gel was left to age for 3 days. After that, the precipitate was separated on a vacuum filter and calcined for 3 hours at 500 °C [1, p. 95].
Fig. 1. Schematic of the laboratory setup
1 ‒ magnetic stirrer; 2 ‒ flask; 3 ‒ reflux condenser
Photocatalytic decomposition of dyes under static conditions. The photocatalytic decomposition of dyes under static conditions in the laboratory was realized as follows. Accurate weights of 0.01 and 0.015 g of photocatalyst were transferred to a 7.5 cm wide and 4.5 cm high chemical beaker, and 15 ml of aqueous dye solution was added. The resulting suspension was then dispersed in an ultrasonic bath for a certain time (2 or 5 min). Next, the suspension was placed on a magnetic stirrer, where it was stirred for 5 or 10 minutes. After that, the sample was irradiated with a UV lamp of 8 or 24 W. The duration of UV irradiation ranged from 5 to 40 minutes. The next step was to filter the suspension using a syringe membrane filter. The absorbance spectrum of the solution obtained after filtration was taken to determine the residual concentration of the dye. Calculations were performed according to the following formulas:
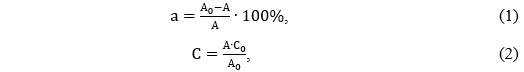
where A0 is the optical density of the original solution; A is the optical density of the resulting solution; C0 ‒ the concentration of the original dye solution (mg/L) [2, p. 539].
Instrumental methods of analysis. The phase composition was identified using a TTR3 Rigaku X-ray powder diffractometer (Japan) with CuKα radiation (λ = 1.5406 Å), 30 mA and 30 kV in a certified RIGAKU laboratory of the Faculty of Engineering and Chemistry of Igor Sikorsky Kyiv Polytechnic Institute.
One of the main characteristics of a semiconductor, which is determined based on the band theory of solids, is the width of the band gap. When optical radiation interacts with a semiconductor, the following types of absorption are distinguished: intrinsic absorption, which corresponds to the transition of the main charge carriers of a semiconductor of this type from the valence band to the conduction band in the absence of impurities; absorption of electromagnetic energy by electrons and holes due to their movement under the action of a light wave electric field. This type of absorption is called plasma absorption. If a semiconductor absorbs radiation and as a result an electron-hole pair is formed, such absorption is called excitonic absorption. In addition to the above, there is also the absorption of radiation energy by vibrations of the crystal lattice and intrinsic absorption in substances with a complex structure of energy bands. All these types of absorption can occur in a single semiconductor and only in a perfectly pure semiconductor. The study of absorption spectra is one of the main methods for determining the characteristics of a semiconductor.
The surface morphology and particle shape of the obtained samples were studied using a scanning electron microscope (SEM) SELMI REM-106I at a voltage of 10 KeV and images of the object were obtained in a wide range of magnifications.
The low-temperature adsorption-desorption isotherm of nitrogen on the synthesized zinc (II) oxide sample was obtained using a Quatachrome Nova 1000e device. To calculate the structural and adsorption characteristics, the data obtained were calculated using known adsorption models: Barrett-Joyner-Halenda (BJH), Brunauer-Emmett-Teller (BET), Horvath-Kawazoe (HK), and density functional theory (DFT).
The BET method allows to calculate the monolayer capacity in the case of polymolecular adsorption. In the experimental determination of the specific surface area, it is convenient to proceed from the number of adsorbed molecules on the surface to their mass [3, p. 55].
Literature:
1. Kumar S., Awasthi K., Kumar Mishra Y. 4 - Synthesis of ZnO nanostructures. Nanostructured Zinc Oxide. 2021. Vol. 4. P. 93‒116.
2. Bhardwaj K., Singh A. K. Bio-waste and natural resource mediated eco-friendly synthesis of zinc oxide nanoparticles and their photocatalytic application against dyes contaminated water. Chemical Engineering Journal Advances. 2023. Vol. 16. P. 536‒548.
3. Omri K., Najeh I., Dhahri R. Effects of temperature on the optical and electrical properties of ZnO nanoparticles synthesized by sol–gel method. Microelectronic Engineering. 2014. Vol. 128. P. 53‒58.
|